A molecule called nidogen-2 may be a key driver of pancreatic cancer progression and metastasis, offering a promising new treatment approach for this aggressive cancer, according to a Garvan Institute study.
Researchers at the Garvan Institute of Medical Research have identified a potential new therapeutic target for pancreatic cancer, one of the most lethal cancer types with limited treatment options. Published in the journal Science Advances, the study shows that blocking the molecule nidogen-2 enhanced the effectiveness of chemotherapy and reduced the cancer’s spread in mouse models.
The team discovered that nidogen-2 reduces the dense scaffolding tissue within pancreatic tumors, which is a major barrier to treatment and contributes to the cancer’s well known chemotherapy resistance.
“Our findings suggest that lowering nidogen-2 could improve the way we treat pancreatic cancer and lead to significantly less metastasis—which is one of the main causes of death in pancreatic cancer,” says Dr. Brooke Pereira, co-first and co-corresponding author of the study and senior research officer at Garvan.
Breaking down tumors to find new targets
Pancreatic cancer is an aggressive disease with a five-year survival rate of just 12{e60f258f32f4d0090826105a8a8e4487cca35cebb3251bd7e4de0ff6f7e40497}, largely because it is often diagnosed at an advanced stage and can resist conventional treatment options.
To identify new therapeutic targets, the Garvan researchers used an innovative technique called tissue decellularization, which removes all the cells from a tumor sample, but retains its scaffolding components, otherwise known as the extracellular matrix.
By comparing the scaffolds of mouse tumors that metastasize with those that don’t, they discovered that the molecule nidogen-2 was elevated in the matrix of more aggressive tumors as the disease progressed.
Using CRISPR gene editing, the researchers then depleted nidogen-2 levels in pancreatic tumors to observe its effect on the cancer’s growth and treatment response in mouse models in real-time, using state-of-the-art intravital imaging at Garvan’s ACRF INCITe Center.
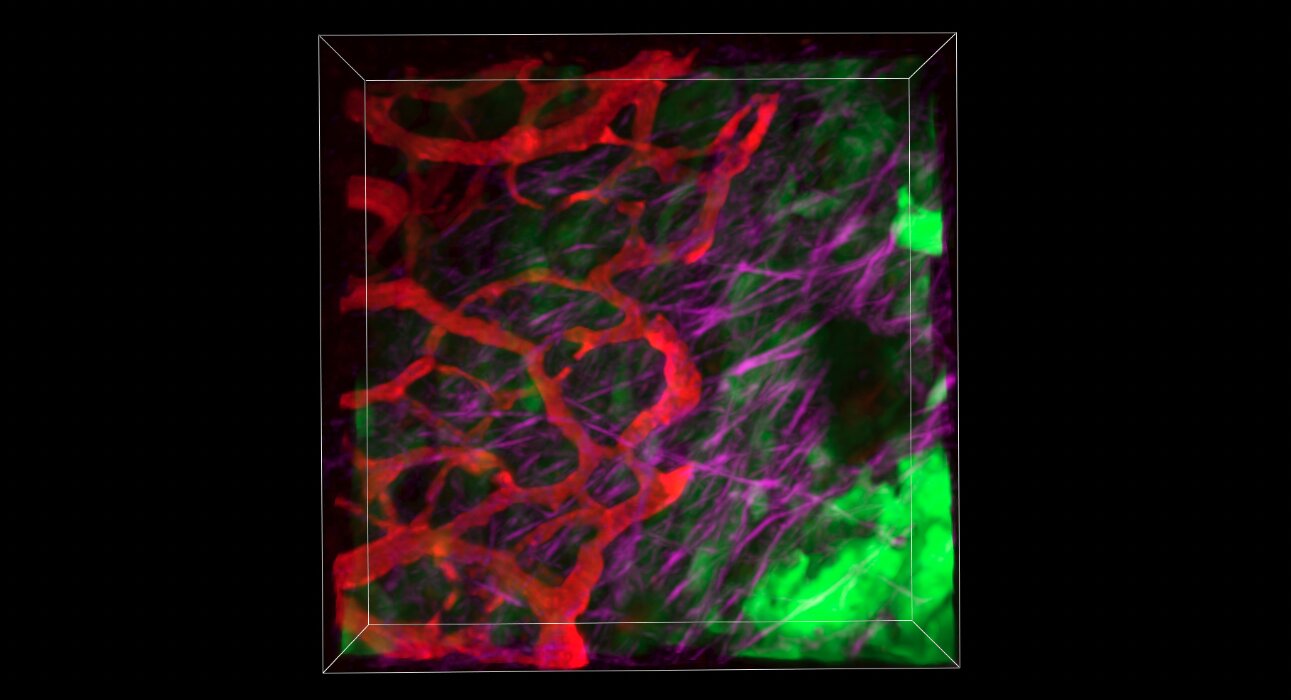
“The results were striking. When we reduced nidogen-2 in pancreatic tumors, we saw a decrease in matrix density, smaller tumors, and an improvement in blood vessel structure,” says Dr. Pereira.
“Tumors with less nidogen-2 had blood vessels that were more open—they were wider and more evenly distributed compared to the collapsed and chaotic vessels usually seen in pancreatic cancer. This caught our attention, because you need functional blood vessels to deliver chemotherapy drugs into the tumor effectively—it’s one of the biggest challenges in treating pancreatic cancer. By targeting nidogen-2, we were able to normalize the tumor blood vessels.”
Improving chemotherapy and reducing metastasis
When researchers administered chemotherapy in their models with reduced levels of nidogen-2, they found that the treatment could more effectively reach the whole tumor.
Nidogen-2 reduction also led to significantly less metastatic spread to the liver in mouse models and improved survival compared to controls.
“This dual effect of enhancing chemotherapy while also reducing metastasis is really exciting. It suggests that targeting nidogen-2 could be a promising new approach for pancreatic cancer,” says Associate Professor Thomas Cox, co-senior author and head of the Matrix and Metastasis Lab at Garvan.
“Our novel approach—removing all the cells from the tumor tissue to leave behind the scaffolding of the tumor—allowed us to identify molecules like nidogen-2 that weren’t previously on our radar,” says Professor Paul Timpson, co-senior author of the study and head of the Invasion and Metastasis Lab at Garvan.
“It’s a powerful way to uncover new clinical targets in the tumor microenvironment—which for decades was overlooked but we now know plays a critical role in cancer progression.”
The researchers are now working on developing clinical approaches to target nidogen-2, such as blocking antibodies that bind to it, which could be combined with existing chemotherapy regimens to allow the drugs to better penetrate the tumor and kill cancer cells.
The researchers say that in future, this approach may also be combined with immunotherapy to further improve outcomes for pancreatic cancer patients. “Pancreatic cancer has seen minimal improvement in survival for decades, so we urgently need new tactics,” says Professor Timpson.
“We believe targeting the tumor scaffolding through nidogen-2 could be a vital step forward in improving treatment of this aggressive disease.”
More information:
Brooke A. Pereira et al, Temporally resolved proteomics identifies nidogen-2 as a cotarget in pancreatic cancer that modulates fibrosis and therapy response, Science Advances (2024). DOI: 10.1126/sciadv.adl1197
Citation:
Promising new target discovered in pancreatic cancer could boost chemotherapy and reduce spread (2024, July 8)
retrieved 8 July 2024
from https://medicalxpress.com/news/2024-07-pancreatic-cancer-boost-chemotherapy.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.