Ontario’s recent decision to eliminate oxycontin and delist its replacement, oxyneo, from the Ontario Drug Benefit program sends a powerful message across the province; that something needs to be done about this epidemic addiction to prescription painkillers.
Ontario’s recent decision to eliminate oxycontin and delist its replacement, oxyneo, from the Ontario Drug Benefit program sends a powerful message across the province; that something needs to be done about this epidemic addiction to prescription painkillers.
Oxyneo is a tablet that is purposely difficult to crush and forms into a thick gel when added to liquid to prevent oxycodone from being extracted for use by injection.
Basically, oxycontin will no longer be manufactured in Canada and it will be replaced with a new formulation called oxyneo by the end of February 2012. Additionally, the Ontario Drug Benefit program will not list oxyneo in their formulary; therefore no coverage will exist for those using this program to fund their habit.
Dr. Irfan Dhalla of Toronto’s St. Michael’s Hospital discovered a spike in opioid-related deaths, which coincided with the addition of long-acting oxycodone to Ontario’s drug plan in 2000.
The Ontario Drug Benefit Program (ODB) offered through the Ministry of Health and Long Term Care (MOHLTC) covers most of the cost of prescription drug products listed in the ODB Formulary. It is available to people 65 years of age and older, residents of long-term care homes and Homes for Special Care, people receiving professional services under the Home Care program, Trillium Drug Program registrants and people in receipt of Ontario Works or Ontario Disability Support Program assistance.
That being said, the drug will still be available to certain individuals through the Exceptional Access Program (EAP). This program facilitates patient access to drugs not funded on the ODB Formulary, or where there is no listed alternative available. To apply for funding through the EAP, a physician must submit a request, which documents all complete and relevant medical information that provides a clinical rationale for requesting coverage including the reason(s) that other similar listed drugs are not suitable. All requests are reviewed according to the guidelines put forth by the ministry’s expert advisory committee, the Committee to Evaluate Drugs (CED), which thoroughly assesses each patient’s specific case and clinical circumstances.
In short, oxycontin will no longer be available commercially in Canada and its replacement, oxyneo, will be under more strict regulations in Ontario so that it is less available to just anyone under the ODB, thereby making it less easily abused.
However, with such great strides comes a myriad of problems when all these individuals begin to experience withdrawal. As major changes are being seen at the provincial level only, neighbouring provinces may have less or no restrictions on oxyneo, which may encourage smuggling. For instance, “Manitoba and British Columbia are among a handful of provinces that have yet to decide whether to fund OxyNeo once OxyContin is discontinued” yet Prince Edward Island and New Brunswick, like Ontario, have chosen not to pay for the new drug. Nevertheless, to avoid such great lengths, addicts may simply turn to other prescription drugs.
There is a high risk of experiencing severe withdrawal symptoms if a patient discontinues oxycodone abruptly. Therefore therapy should be discontinued gradually rather than abruptly. People who use oxycodone in a hazardous or harmful fashion are at even higher risk of severe withdrawal symptoms as they tend to use higher than prescribed doses. The symptoms of oxycodone withdrawal are the same as for other opiate based painkillers and may include “anxiety, panic attack, nausea, insomnia, muscle pain, muscle weakness, fevers, and other flu like symptoms.”
Clearly prohibiting this drug in itself will not fix the problem. Perhaps the savings from delisting this drug could be more appropriately used to fund treatment programs across the province…
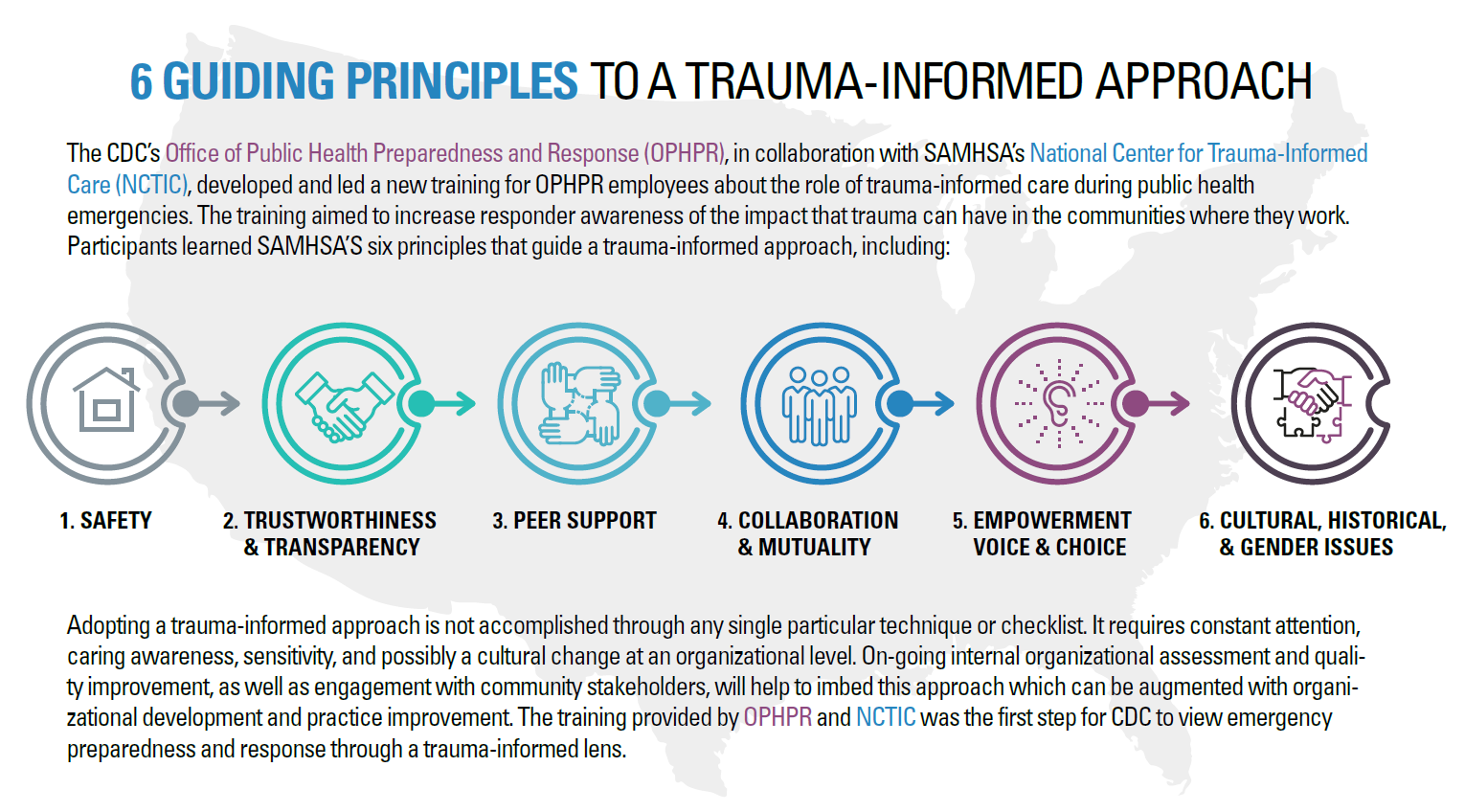
OxyContin limits lauded
Exceptional Access Program (EAP)
Ontario Drug Benefit: The Program
Oxycodone
© www.understandingaddictions.com
SOURCE: Understanding Addictions – Read entire story here.