Novel research, presented today at the European Academy of Dermatology and Venereology (EADV) Congress 2024, has identified key molecular targets that could significantly enhance the healing of both acute and chronic wounds.
These findings represent a crucial advancement in wound care, paving the way for more effective treatment options and improved patient outcomes.
Globally, acute and chronic wounds affect nearly one billion people. In particular, chronic wounds pose a substantial economic burden on health care systems and severely impact the quality of life for those affected. Despite this, current treatment strategies are often limited, highlighting an urgent need for a deeper understanding of the mechanisms underlying impaired wound healing.
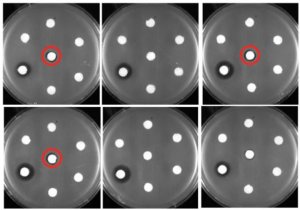
To address this, researchers conducted a study using healthy full-thickness human skin punches, creating central, partial wounds. These samples were then cultured under either physiological or pathological conditions, including hyperglycemia, oxidative stress and hypoxia, to mimic acute and chronic wounds, respectively. Using advanced comparative transcriptomic profiling with bulk RNA sequencing, the team monitored gene expression changes over a five-day period.
The results revealed several critical differences in gene activity between acute and chronic wounds. Key wound repair-associated genes such as KRT6A-C, PTX3, KRT1, KRT10, COL1A1, along with pathways including Wnt signaling and actin cytoskeleton organization, were differentially regulated between acute and chronic wounds.
Additionally, overall gene expression was downregulated in chronic wounds compared to acute wounds, suggesting that essential genes required for effective wound healing are inadequately transcribed in these conditions.
Notably, FGF7, a key promoter of epithelial cell proliferation and tissue repair, was significantly downregulated in chronic wounds by Day 5. In contrast, MMP10, a tissue-degrading enzyme, was elevated throughout the study period in chronic wounds.
To counteract these imbalances, the researchers tested the effects of recombinant FGF7 protein and an MMP10-neutralizing antibody (α-MMP10) on acute and chronic wounds in the ex vivo wound models. Topical administration of α-MMP10 led to a significant increase in wound tongue length, indicating improved healing in acute wounds. In contrast, FGF7 did not show a significant effect on its own.
The combined application of FGF7 and α-MMP10, however, significantly enhanced re-epithelization in both types of wounds.
“While we must be cautious when discussing synergistic effects, our preliminary data reveal that combinatorial therapy may be a valid option for treating chronic wounds,” explains Dr. Marta Bertolini, lead author of the study and Managing Director of QIMA Monasterium GmbH. “We believe that administering excessive FGF7 promotes epidermal keratinocyte proliferation and mobilization, which are crucial for wound healing. At the same time, neutralizing MMP10 removes a barrier to keratinocyte movement, potentially accelerating re-epithelization.”
The study also identified osteopontin (SPP1) as a gene significantly upregulated on Days 3 and 5 in acute wounds compared to chronic wounds. To leverage this finding, the researchers administered FOL005, an osteopontin-derived peptide, to experimentally induced wounds ex vivo.
Treatment with FOL005 significantly enhanced skin re-epithelization under both physiological and pathological conditions, highlighting its potential as an effective therapeutic option for acute and chronic wound management.
“We believe these findings mark a significant step forward in understanding the complex biology of wound healing,” concludes Dr. Bertolini. “Our transcriptomic data will soon be accessible, and we hope this will inspire other researchers and industry to identify additional promising targets that could offer much-needed relief to patients affected by these challenging and often debilitating wounds.”
More information:
Piccini, I., Fehrholz, M., Ludwig, R., Zibert, J., Alenfall, J., Hultgårdh Nilsson, A., Nilsson, J., Paus, R., & Bertolini, M. (2024). Using comparative transcriptomic profiling ex vivo to identify novel, potential targets for acute and chronic wound healing. Presented at the European Academy of Dermatology and Venereology (EADV) Congress 2024.
Provided by
European Academy of Dermatology and Venereology
Citation:
Key molecular targets for wound healing identified (2024, September 25)
retrieved 26 September 2024
from https://medicalxpress.com/news/2024-09-key-molecular-wound.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.