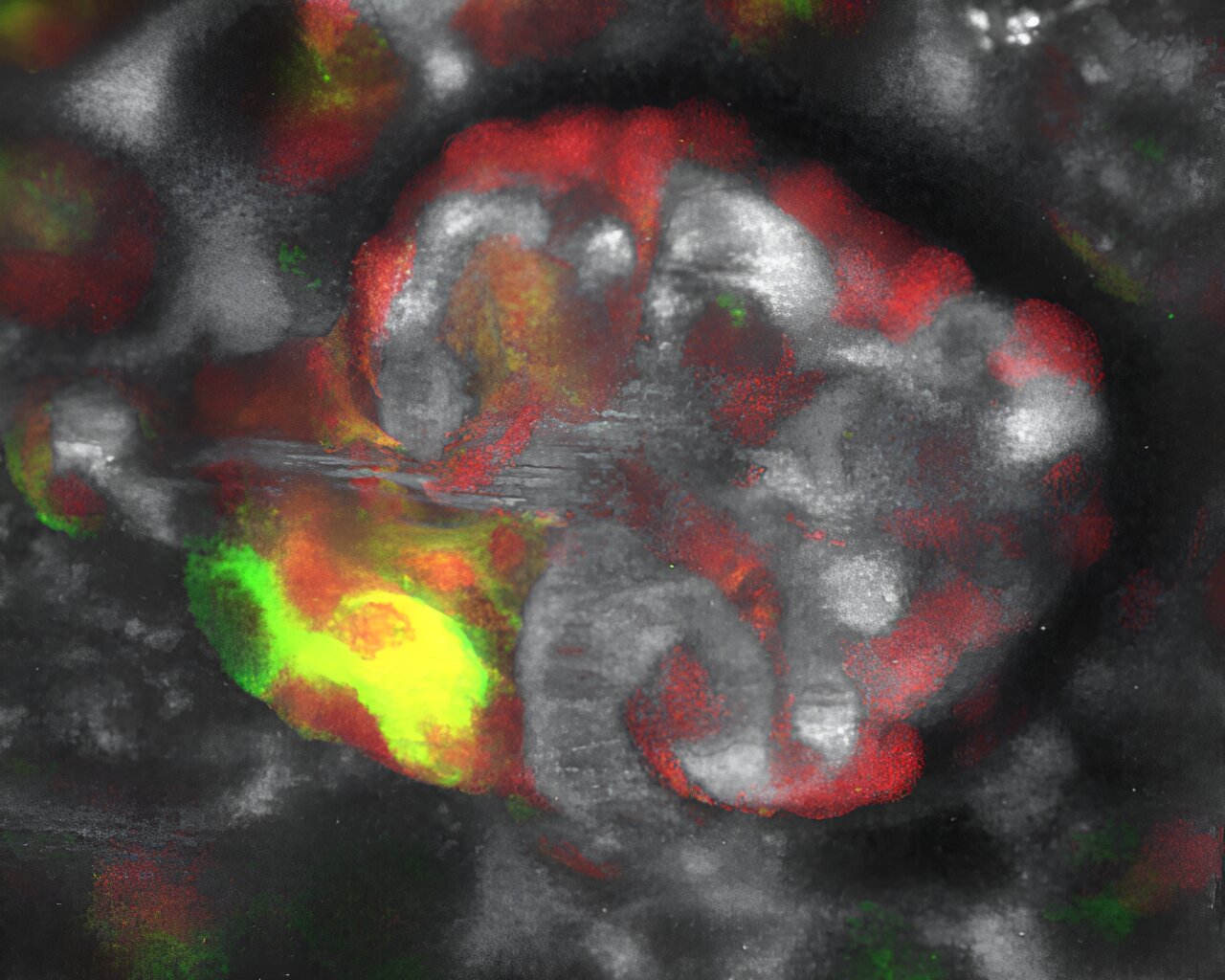
A loss of salt and body fluid can stimulate kidney regeneration and repair in mice, according to a new study led by USC Stem Cell scientist Janos Peti-Peterdi and published in the Journal of Clinical Investigation.
This innate regenerative response relies on a small population of kidney cells in a region known as the macula densa (MD), which senses salt and exerts control over filtration, hormone secretion, and other key functions of this vital organ.
“Our personal and professional mission is to find a cure for kidney disease, a growing global epidemic affecting one 1 of 7 adults, which translates to 850 million people worldwide or about 2 million in the Los Angeles area,” said Peti-Peterdi, a professor of physiology, neuroscience and medicine at the Keck School of Medicine of USC.
“Currently, there is no cure for this silent disease. By the time kidney disease is diagnosed, the kidneys are irreversibly damaged and ultimately need replacement therapies, such as dialysis or transplantation.”
To address this growing epidemic, Peti-Peterdi, first author Georgina Gyarmati, and their colleagues took a highly non-traditional approach. As opposed to studying how diseased kidneys fail to regenerate, the scientists focused on how healthy kidneys originally evolved.
“From an evolutionary biology perspective, the primitive kidney structure of the fish turned into more complicated and more efficiently working kidneys to absorb more salt and water,” said Peti-Peterdi, who also directs the Multi-Photon Microscopy Core at the Zilkha Neurogenetic Institute (ZNI).
“This was necessary for adaptation to the dry land environment when the animal species moved from the salt-rich seawater. And that’s why birds and mammals have developed MD cells and this beautiful, bigger, and more efficient kidney structure to maintain themselves and functionally adapt to survive. These are the mechanisms that we are targeting and trying to mimic in our research approach.”
With this evolutionary history in mind, the research team fed lab mice a very low salt diet, along with a commonly prescribed drug called an ACE inhibitor that furthered lowered salt and fluid levels. The mice followed this regimen for up to two weeks, since extremely low salt diets can trigger serious health problems if continued long term.
In the region of the MD, the scientists observed regenerative activity, which they could block by administering drugs that interfered with signals sent by the MD. This underscored the MD’s key role in orchestrating regeneration.
When the scientists furthered analyzed mouse MD cells, they identified both genetic and structural characteristics that were surprisingly similar to nerve cells. This is an interesting finding, because nerve cells play a key role in regulating the regeneration of other organs such as the skin.
In the mouse MD cells, the scientists also identified specific signals from certain genes, including Wnt, NGFR, and CCN1, which could be enhanced by a low-salt diet to regenerate kidney structure and function. In keeping with these findings in mice, the activity of CCN1 was found to be greatly reduced in patients with chronic kidney disease (CKD).
To test the therapeutic potential of these discoveries, the scientists administered CCN1 to mice with a type of CKD known as focal segmental glomerulosclerosis. They also treated these mice with MD cells grown in low-salt conditions.
Both approaches were successful, with the MD cell treatment producing the biggest improvements in kidney structure and function. This might be due to the MD cells secreting not only CCN1, but also additional unknown factors that promote kidney regeneration.
“We feel very strongly about the importance of this new way of thinking about kidney repair and regeneration,” said Peti-Peterdi. “And we are fully convinced that this will hopefully end up soon in a very powerful and new therapeutic approach.”
More information:
Georgina Gyarmati et al, Neuronally differentiated macula densa cells regulate tissue remodeling and regeneration in the kidney, Journal of Clinical Investigation (2024). DOI: 10.1172/JCI174558
Citation:
Loss of salt and body fluid stimulates kidney regeneration in mice (2024, June 28)
retrieved 28 June 2024
from https://medicalxpress.com/news/2024-06-loss-salt-body-fluid-kidney.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.